Data requests
Who can request data
Researchers belonging to IDIAP-accredited research groups or ICS primary care researchers will be eligible for a preferential rate.
Research projects proposed by research groups accredited by the IDIAP Jordi Gol will be given top priority by SIDIAP.
Researchers from research groups of other public research institutions can also apply for data to SIDIAP.
The regulatory framework that will govern access to SIDIAP data by these groups and/or institutions will be established through the signing of an agreement. Various aspects (including data security) will be taken into account for establishing the conditions of access to the data.
SIDIAP does not cede data to for-profit entities, but it can carry out quality research projects and deliver the relevant results reports at the end of the project, as long as these projects are required by a Regulatory Agency, such as the European Medicine Agency (EMA) or the Food and Drug Administration (FDA).
European projects will also be accepted if, although they are funded by non-profit organisations, there is public participation and a public call for proposals, such as the Innovative Medicines Initiative (IMI) projects.
In an initial phase, an IDIAP research team will be sought, and this will be responsible for defining the design of the study together with the external entity. Once the protocol has been finalised (and approved with the necessary changes by the SIDIAP and Ethics CC), the research team will carry out the project and deliver the different reports established in the agreement previously signed between the IDIAP Jordi Gol and the external entity. These studies will be subject to the rules of conduct dictated by the ENCEPP.
Key elements of collaboration
- Use of data exclusively for research projects.
- The objectives of the project cannot be contrary to the health policy of the Catalan Institute of Health.
- The ethics committees and governing bodies must have approved the studies before they begin.
- Unprocessed data will not be transferred to the sponsor of the study. The IDIAP is responsible for the design of the study and data analysis. A report is issued at the end of the study.
- Freedom to carry out studies for other entities.
- Freedom of publication. Scientific independence in accordance with the ENCEPP Code.
- Limitation of liability to the total amount of each study.
- Intellectual Property Rights. The IDIAP will maintain intellectual property or, by default, reserves the right to use the results for internal use for non-commercial research or teaching purposes.
- Delivery of a copy of the Report to the Institut Català de la Salut for internal use.
Data request circuit
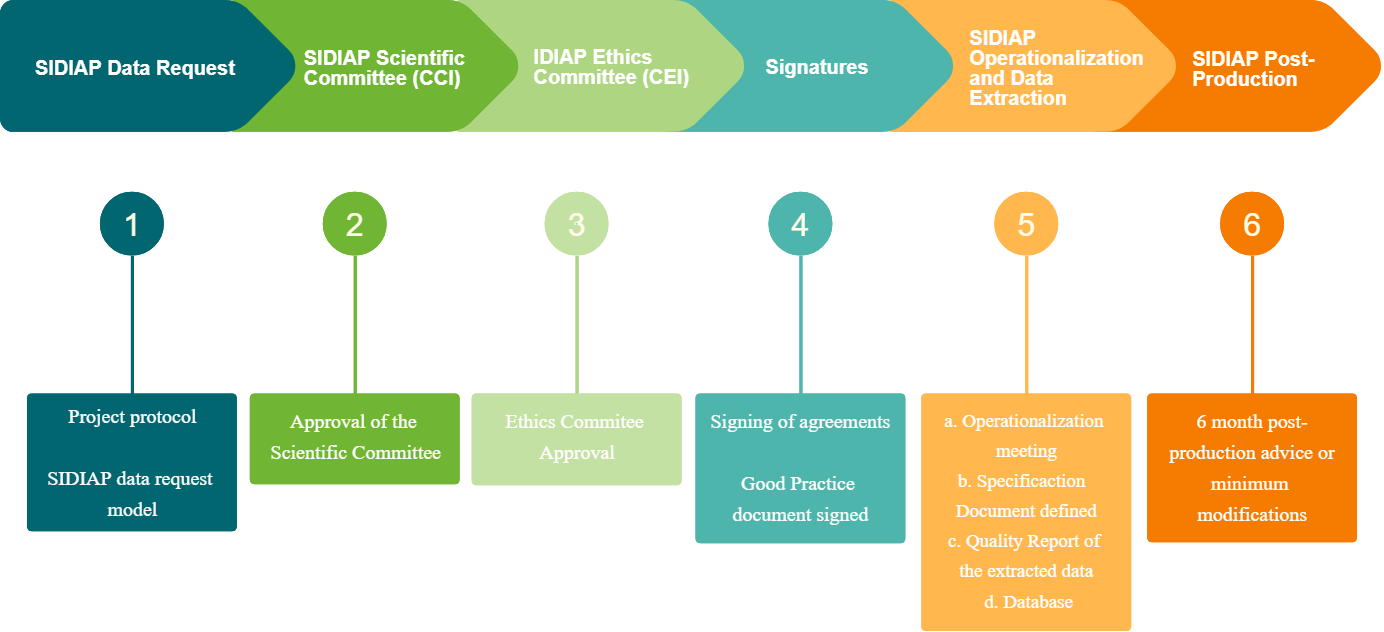
SIDIAP Application
The Application Form must be sent by e-mail to This email address is being protected from spambots. You need JavaScript enabled to view it., and the study protocol must be attached.
It is recommended to consult the guide to help you with the Content of a Research Protocol.
For more useful information, please consult the section Other Resources for Researchers..
The Scientific Committee meets once a month during the first week of the month and the deadline for receiving applications is the 15th of the previous month.
Scientific Committee Approval
The Scientific Committee meets monthly on the first Thursday of each month. If the evaluation is Positive: the protocol will be sent to the CEIC for evaluation. If clarifications are requested: these will be sent by e-mail to the Principal Investigator and/or the person who managed the application. The project will have to be resubmitted identifying the modifications made with track changes and attaching a letter of response to the reviewers. The project will be re-evaluated at the next meeting of the Committee. The deadline for receiving clarifications is 1 week before the meeting. If the evaluation is negative: the Principal Investigator will be notified of the reasons for the refusal by e-mail.
Ethics Committee approval
Every project done with the SIDIAP also needs the approval of the Ethics Committee. Once the project has been approved by the SIDIAP Scientific Committee, the SIDIAP Technical Secretariat will send it to the REC for final approval.
Signing of Agreements
Once the project has been approved by the SIDIAP SC and the REC, the Principal Investigator will be notified and will be sent the Document of Good Practices in the use of SIDIAP data, which he/she will have to return signed and scanned.
If the person in charge of the project is from an external entity, a collaboration agreement must be signed with the corresponding entity.
Having these two documents signed is a prerequisite to continue with the next steps.
Production / Data Extraction
An initial meeting will be held with the PI of the project or research team and the SIDIAP technicians to resolve doubts and begin the operationalisation of the protocol, i.e. to transform the epidemiological protocol into a series of highly parameterised queries, both at the level of variables and time windows, which can be understood by the database.
Once this operationalisation is done, it will be possible to perform the extraction of the database, which will be sent to the IP or to the Data Manager of the project for processing.
Post-production and Protocol Amendments
Post-production: : The research team has 6 months to review and check the data. Corrections during this period may have some priority over the rest of the projects being worked on.
After this time, any correction made to data and/or variables will have to be approved by the Scientific Committee, and the project will be put in the waiting list of ongoing projects.
Protocol amendments: : If, once the project has started, changes must be made to the protocol (changes in the methodology, objectives, analysis, etc.), the CC SIDIAP must be notified through the Amendments Model and, if necessary, the new version of the protocol must be attached, identifying the changes made.
How to request data
All protocols submitted to SIDIAP must contain the following sections:
- Title
- Principal Investigator (name, e-mail, institution)
- Summary of the study
- Keywords
- Title and Abstract in English
- Background, Rationale and Bibliography
- Hypothesis
- General Aims
- Specific Aims
- Methodology
- Design
- Scope and period of study
- Reference population
- Study population with inclusion and exclusion criteria
- Sample size and sampling procedure
- Variables (differentiating between dependent and independent variables)
- Data collection and sources of information
- Data analysis
- Difficulties and limitations
- Ethical considerations and data confidentiality
- Additional procedures resulting from the study (explain in detail the procedures performed on the study participants, which are not standard care practice) (if applicable)
- Work plan (tasks, milestones, and timeline)
- Expertise of the research team regarding the subject matter
- Scientific and technical background
- Applicability and practical usefulness of the results of the study
- Means available to carry out the project
- Justification for funding and budget requested
- Possible conflicts of interest
- Annexes (if applicable)
To ensure that the protocols submitted for evaluation contain the necessary information, a guide has been prepared, , “Content of a Research Protocol with SIDIAP”, which includes all the aspects considered in the “STROBE” (observational studies), RECUERDO (observational studies with large databases) and RECUERDO-PE (pharmacoepidemiological studies with large databases) guides. In the document you will find links to other guidelines that help to design more specific types of studies.